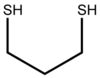
1,3-Propanedithiol
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Propane-1,3-dithiol
|
|
| Other names
1,3-Dimercaptopropane
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.371 |
| RTECS number | TZ2585500 |
|
|
|
|
| Properties | |
| C3H8S2 | |
| Molar mass | 108.22 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.078 g/cm3 |
| Melting point | −79 °C (−110 °F; 194 K) |
| Boiling point | 169 °C (336 °F; 442 K) |
| slight | |
| Solubility in solvents | all organic solvents |
|
Refractive index (nD)
|
1.539 |
| Structure | |
| 0 D | |
| Hazards | |
| Main hazards | stench |
| R-phrases | R36/37/38 |
| S-phrases | S26 |
| Flash point | 138 °C (280 °F; 411 K) |
| Related compounds | |
|
Related compounds
|
1,2-ethanedithiol 1,2-propanedithiol lipoic acid |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
1,3-Propanedithiol is the chemical compound with the formula HSCH2CH2CH2SH. This dithiol is a useful reagent in organic synthesis. This liquid, which is readily available commercially, has an intense stench.
1,3-Propanedithiol is mainly used for the protection of aldehydes and ketones via their reversible formation of dithianes. A prototypical reaction is its formation of 1,3-dithiane from formaldehyde. The reactivity of this dithiane illustrates the concept of umpolung.
The unpleasant odour of 1,3-propanedithiol has encouraged the development of alternative reagents that generate similar derivatives.
1,3-Propanedithiol is used in the synthesis of tiapamil.
1,3-Propanedithiol reacts with metal ions to form chelate rings. Illustrative is the synthesis of the derivative diiron propanedithiolate hexacarbonyl upon reaction with triiron dodecacarbonyl:
The stench of 1,3-propanedithiol can be neutralized with bleach.
...
Wikipedia
