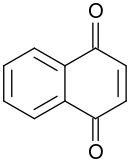
1,4-naphthoquinone
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Naphthalene-1,4-dione
|
|
| Other names
1,4-Naphthoquinone
Naphthoquinone α-Naphthoquinone |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.004.526 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C10H6O2 | |
| Molar mass | 158.15 g/mol |
| Density | 1.422 g/cm3 |
| Melting point | 126 °C (259 °F; 399 K) |
| Boiling point | Begins to sublime at 100 °C |
| 0.09 g/L | |
| -73.5·10−6 cm3/mol | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
1,4-Naphthoquinone or para-naphthoquinone is an organic compound derived from naphthalene. Several isomeric naphthoquinones are known, notably 1,2-naphthoquinone. 1,4-Naphthoquinone forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit.
The industrial synthesis involves aerobic oxidation of naphthalene over a vanadium oxide catalyst:
In the laboratory, naphthoquinone can be produced by the oxidation of a variety of naphthalene compounds. An inexpensive route involves oxidation of naphthalene with chromium trioxide.
1,4-Naphthoquinone acts as strong dienophile in Diels-Alder reaction. Its adduct with 1,3-butadiene can be prepared by two methods: 1) long (45 days) exposure of naphthoquinone in neat liquid butadiene taken in huge excess at room temperature in a thick-wall glass tube or 2) fast catalyzed cycloaddition at low temperature in the presence of 1 equivalent of tin(IV) chloride:
1,4-Naphthoquinone is mainly used as a precursor to anthroquinone by reaction with butadiene followed by oxidation. Nitration gives 5-nitro-1,4-naphthalenedione, precursor to an aminoanthroquinone that is used as a dye precursor.
Naphthoquinone forms the central chemical structure of many natural compounds, most notably the K vitamins. 2-Methylnaphthoquinone is a more effective coagulant than vitamin K.
Other natural naphtoquinones include juglone, plumbagin, droserone.
...
Wikipedia
