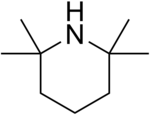
2,2,6,6-Tetramethylpiperidine
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
2,2,6,6-Tetramethylpiperidine
|
|||
| Other names
Norpempidine
Tetramethylpiperidine |
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| Abbreviations | TMP | ||
| ChemSpider | |||
| ECHA InfoCard | 100.011.090 | ||
| EC Number | 212-199-3 | ||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| C9H19N | |||
| Molar mass | 141.254 g/mol | ||
| Appearance | Clear liquid | ||
| Density | 0.83 g/mL | ||
| Melting point | −59 °C (−74 °F; 214 K) | ||
| Boiling point | 152 °C (306 °F; 425 K) | ||
| Hazards | |||
| R-phrases (outdated) | R10 R22 R36/37/38 | ||
| S-phrases (outdated) | S16 S26 S37/39 | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
2,2,6,6-Tetramethylpiperidine or TMP or HTMP is an organic compound of the amine class. In appearance, it is a colorless liquid and has a "fishy", amine-like odor. This amine is used in chemistry as a hindered base (hindered amine). Although TMP finds limited use per se, its derivatives are a mainstay of hindered amine light stabilizers.
TMP is the starting material for an even stronger base lithium tetramethylpiperidide and the radical species TEMPO. Another non-nucleophilic base is N,N-diisopropylethylamine.
Many routes for the synthesis of TMP have been reported. One method starts with a conjugate addition reaction of ammonia to phorone. The intermediate triacetone amine is then reduced in a Wolff-Kishner reaction.
...
Wikipedia