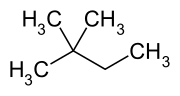
2,2-Dimethylbutane
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
2,2-Dimethylbutane
|
|||
| Other names
Neohexane, 22DMB
|
|||
| Identifiers | |||
|
75-83-2 |
|||
| 3D model (Jmol) | Interactive image | ||
| 1730736 | |||
| ChEMBL |
ChEMBL142735 |
||
| ChemSpider |
6163 |
||
| ECHA InfoCard | 100.000.825 | ||
| EC Number | 200-906-8 | ||
| PubChem | 6403 | ||
| RTECS number | EJ9300000 | ||
| UNII |
07L56L3MP2 |
||
| UN number | 1208 | ||
|
|||
|
|||
| Properties | |||
| C6H14 | |||
| Molar mass | 86.18 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Odorless | ||
| Density | 649 mg mL−1 | ||
| Melting point | −102 to −98 °C; −152 to −145 °F; 171 to 175 K | ||
| Boiling point | 49.7 to 49.9 °C; 121.4 to 121.7 °F; 322.8 to 323.0 K | ||
| log P | 3.51 | ||
| Vapor pressure | 36.88 kPa (at 20 °C) | ||
|
Henry's law
constant (kH) |
6.5 nmol Pa−1 kg−1 | ||
| -76.24·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.369 | ||
| Thermochemistry | |||
| 189.67 J K−1 mol−1 | |||
|
Std molar
entropy (S |
272.00 J K−1 mol−1 | ||
|
Std enthalpy of
formation (ΔfH |
−214.4–−212.4 kJ mol−1 | ||
|
Std enthalpy of
combustion (ΔcH |
−4.1494–−4.1476 MJ mol−1 | ||
| Hazards | |||
| GHS pictograms |
   
|
||
| GHS signal word | DANGER | ||
| H225, H304, H315, H336, H411 | |||
| P210, P261, P273, P301+310, P331 | |||
|
EU classification (DSD)
|
|
||
| R-phrases | R11, R38, R51/53, R65, R67 | ||
| S-phrases | (S2), S16, S29, S33 | ||
| NFPA 704 | |||
| Flash point | −29 °C (−20 °F; 244 K) | ||
| 425 °C (797 °F; 698 K) | |||
| Explosive limits | 1.2–7.7% | ||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
none | ||
| Related compounds | |||
|
Related alkanes
|
|||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
2,2-Dimethylbutane, trivially known as neohexane, is an organic compound with formula C6H14 or (H3C-)3-C-CH2-CH3. It is therefore an alkane, indeed the most compact and branched of the hexane isomers — the only one with a quaternary carbon and a butane (C4) backbone. It can be synthesised by the hydroisomerisation of 2,3-dimethylbutane using an acid catalyst.
...
Wikipedia



