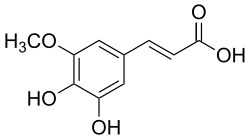
5-hydroxyferulic acid
 |
|
| Names | |
|---|---|
|
IUPAC name
(E)-3-(3,4-dihydroxy-5-methoxyphenyl)prop-2-enoic acid
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.230.072 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C10H10O5 | |
| Molar mass | 210.18 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
5-Hydroxyferulic acid is a hydroxycinnamic acid.
It is a precursor in the biosynthesis of sinapic acid. Phenylalanine is first converted to cinnamic acid by the action of the enzyme phenylalanine ammonia-lyase (PAL). A series of enzymatic hydroxylations and methylations leads to coumaric acid, caffeic acid, ferulic acid, 5-hydroxyferulic acid and sinapic acid.
Thus 5-hydroxyferulic acid is formed from ferulic acid by the action of the specific enzyme ferulate 5-hydroxylase (F5H).
...
Wikipedia
