Adenosine triphosphoric acid
 |
|
| Identifiers | |
|---|---|
|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.258 |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C10H16N5O13P3 | |
| Molar mass | 507.18 g/mol |
| Density | 1.04 g/cm3 (disodium salt) |
| Melting point | 187 °C (369 °F; 460 K) disodium salt; decomposes |
| Acidity (pKa) | 6.5 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Adenosine triphosphate (ATP) is a complex organic chemical that participates in many processes. Found in all forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts to either the di- or monophosphates, respectively ADP and AMP. Other processes regenerate ATP such that the human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA.
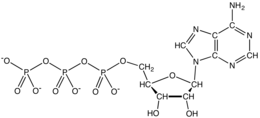
From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components, a nitrogenous base (adenine), the sugar ribose, and the triphosphate. It is used in cells as a coenzyme.
In terms of its structure, ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar (ribose), which in turn is attached at the 5′ carbon atom of the sugar to a triphosphate group. In its many reactions related to metabolism, the adenine and sugar groups remain unchanged, but the triphosphate is converted to di- and monophosphate, giving respectively the derivatives ADP and AMP. The three phosphoryl groups are referred to as the alpha (α), beta (β), and, for the terminal phosphate, gamma (γ).
In neutral solution, ionized ATP exists mostly as ATP4−, with a small proportion of ATP3−.
Being polyanionic and featuring a potentially chelatable polyphosphate group, ATP binds metal cations with high affinity. The binding constant for Mg2+
is (9554). The binding of a divalent cation, almost always magnesium, strongly affects the interaction of ATP with various proteins. Due to the strength of the ATP-Mg2+ interaction, ATP exists in the cell mostly as a complex with Mg2+
bonded to the phosphate oxygen centers.
...
Wikipedia
