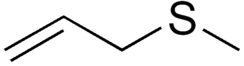
Allyl methyl sulfide
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
3-Methylsulfanylprop-1-ene
|
|
| Other names
Methyl propenyl sulfide
3-Methylthio-1-propene |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.371 |
| EC Number | 233-422-0 |
| MeSH | allyl+methyl+sulfide |
|
PubChem CID
|
|
| RTECS number | UD1015000 |
| UNII | |
| UN number | 1993 |
|
|
|
|
| Properties | |
| C4H8S | |
| Molar mass | 88.17 g·mol−1 |
| Odor | Garlic |
| Density | 0.803 g cm−3 |
| Boiling point | 92 °C; 197 °F; 365 K |
| Hazards | |
| GHS pictograms |  |
| GHS signal word | DANGER |
| H225 | |
| P210 | |
| Flash point | 18.0 °C (64.4 °F; 291.1 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Allyl methyl sulfide (AMS) is an organosulfur compound with the chemical formula CH2=CHCH2SCH3. The molecule features two functional groups, an allyl (CH2=CHCH2) and a sulfide. It is a colourless liquid with a strong odor characteristic of alkyl sulfides. It is a metabolite of garlic, and "garlic breath" is attributed to its presence.
It is prepared by the reaction of allyl chloride with sodium hydroxide and methanethiol.
...
Wikipedia
