Bexarotene
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Targretin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608006 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral and topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | >99% |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Biological half-life | 7 hours |
| Excretion | Parent drug and metabolites are eliminated primarily through the hepatobiliary system. Less than 1% is excreted in the urine unchanged. |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.206.790 |
| Chemical and physical data | |
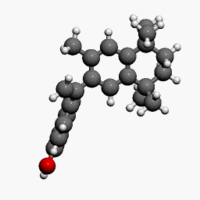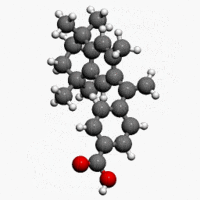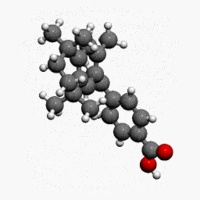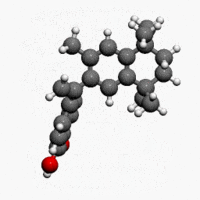
| Formula | C24H28O2 |
| Molar mass | 348.478 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Bexarotene (brand name: Targretin) is an antineoplastic (anti-cancer) agent approved by the U.S.Food and Drug Administration (FDA) (in late 1999) and the European Medicines Agency (EMA) (early 2001) for use as a treatment for cutaneous T cell lymphoma (CTCL). It is a third-generation retinoid.
Bexarotene is indicated for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma in people who are refractory to at least one prior systemic therapy (oral) and for the topical treatment of cutaneous lesions in patients with CTCL who have refractory or persistent disease after other therapies or who have not tolerated other therapies (topical).
It has been used off-label for non-small cell lung cancer and breast cancer.
Known contraindications include:
Overall the most common adverse effects are skin reactions (mostly itchiness and rashes), leucopenia, headache, weakness, thyroid anomalies (which seem to be mediated by RXR-mediated downregulation of thyroid stimulating hormone) and blood lipid anomalies such as hypercholesterolaemia (high blood cholesterol) and hyperlipidaemia.
Its plasma concentration may be increased by concomitant treatment with CYP3A4 inhibitors such as . It may also induce CYP3A4, and hence CYP3A4 substrates like cyclophosphamide may have their plasma concentrations reduced. Likewise consumption of grapefruit juice might increase bexarotene's plasma concentrations, hence potentially altering its therapeutic effects.
Bexarotene is a retinoid that selectively activates retinoid X receptors (RXRs), as opposed to the retinoic acid receptors, the other major target of retinoic acid (the acid form of vitamin A). By so doing it induces cell differentiation and apoptosis and prevents the development of drug resistance. It also has anti-angiogenic effects and inhibits cancer metastasis. The retinoic acid receptors (RARs) regulate cell differentiation and proliferation whereas RXRs regulate apoptosis.
...
Wikipedia
