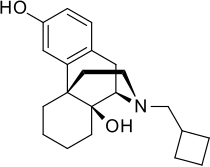
Butorphanol
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Stadol |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a682667 |
| Pregnancy category |
|
| Routes of administration |
IV, intranasal, oral |
| ATC code | N02AF01 (WHO) QR05DA90 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Nasal: 60-70% |
| Metabolism | Hepatic hydroxylated & glucuronidated |
| Biological half-life | 4-7 hours |
| Excretion |
Renal, 75% Biliary, 11-14% Fecal, 15% |
| Identifiers | |
|
|
| CAS Number |
42408-82-2 |
| PubChem (CID) | 5361092 |
| IUPHAR/BPS | 7591 |
| DrugBank |
DB00611 |
| ChemSpider |
16735714 |
| UNII |
QV897JC36D |
| KEGG |
D00837 |
| ChEBI |
CHEBI:3242 |
| ChEMBL |
CHEMBL33986 |
| ECHA InfoCard | 100.050.717 |
| Chemical and physical data | |
| Formula | C21H29NO2 |
| Molar mass | 327.473 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
Butorphanol (AAN, BAN, INN and USAN) is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers. Brand name Stadol was recently discontinued by the manufacturer. It is now only available in its generic formulations, manufactured by Novex, Mylan, Apotex and Ben Venue Laboratories. Butorphanol is most closely structurally related to levorphanol. Butorphanol is available as the tartrate salt in injectable, tablet, and intranasal spray formulations. The tablet form is only used in dogs and cats due to low bioavailability in humans.
Butorphanol is listed under the Single Convention on Narcotic Drugs 1961 and in the United States is a Schedule IV Narcotic controlled substance with a DEA ACSCN of 9720; being in Schedule IV it is not subject to annual aggregate manufacturing quotas. The free base conversion ratio of the hydrochloride is 0.69. Butorphanol was originally in Schedule II and at one point it was decontrolled. Butorphanol derived from thebaine was scheduled separately as Schedule II, then decontrolled on 14. July 1992 and put in Schedule IV on 31. October 1997.
Butorphanol exhibits partial agonist and antagonist activity at the μ-opioid receptor, as well as partial agonist activity at the κ-opioid receptor (Ki = 2.5 nM; EC50 = 57 nM; Emax = 57%). Stimulation of these receptors on central nervous system neurons causes an intracellular inhibition of adenylate cyclase, closing of influx membrane calcium channels, and opening of membrane potassium channels. This leads to hyperpolarization of the cell membrane potential and suppression of action potential transmission of ascending pain pathways. Because of its κ-agonist activity, at analgesic doses butorphanol increases pulmonary arterial pressure and cardiac work. Additionally, κ-agonism can cause dysphoria at therapeutic or supertherapeutic doses; this gives butorphanol a lower potential for abuse than other opioid drugs.
...
Wikipedia
