Cabazitaxel
 |
|
| Clinical data | |
|---|---|
| Trade names | Jevtana |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a611009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.205.741 |
| Chemical and physical data | |
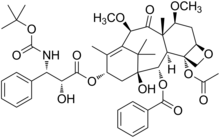
| Formula | C45H57NO14 |
| Molar mass | 835.93 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Cabazitaxel (previously XRP-6258, trade name Jevtana) is a semi-synthetic derivative of a natural taxoid. It was developed by Sanofi-Aventis and was approved by the U.S. FDA for the treatment of hormone-refractory prostate cancer on June 17, 2010. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.
Cabazitaxel in combination with prednisone is a treatment option for hormone-refractory prostate cancer following docetaxel-based treatment.
In a phase III trial with 755 men for the treatment of castration-resistant prostate cancer, median survival was 15.1 months for patients receiving cabazitaxel versus 12.7 months for patients receiving mitoxantrone. Cabazitaxel was associated with more grade 3–4 neutropenia (81.7%) than mitoxantrone (58%).
...
Wikipedia
