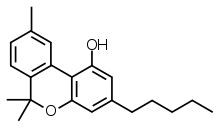
Cannabinol
 |
|
 |
|
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number |
521-35-7 |
| PubChem (CID) | 2543 |
| IUPHAR/BPS | 740 |
| ChemSpider |
2447 |
| UNII |
7UYP6MC9GH |
| KEGG |
C07580 |
| ChEMBL |
CHEMBL74415 |
| ECHA InfoCard | 100.216.772 |
| Chemical and physical data | |
| Formula | C21H26O2 |
| Molar mass | 310.4319 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 77 °C (171 °F) |
| Solubility in water |
insoluble in water soluble in methanol and ethanol mg/mL (20 °C) |
|
|
|
|
|
|
|
insoluble in water
Cannabinol (CBN) is a weak psychoactive cannabinoid found only in trace amounts in Cannabis sativa and Cannabis indica. Pharmacologically relevant quantities are formed as a metabolite of tetrahydrocannabinol (THC) . CBN acts as a partial agonist at the CB1 receptors, but has a higher affinity to CB2 receptors, however; with lower affinities in comparison to THC. Degraded or oxidized cannabis products, such as low-quality baled cannabis and traditionally produced hashish, are high in CBN, but modern production processes minimize the formation of CBN. Cannabinol has been shown to have analgesic properties.
Unlike other cannabinoids, CBN does not stem from cannabigerol (CBG) but rather is the degraded product of THC. If cannabis is exposed to air or ultraviolet light (for example, in sunlight) for a prolonged period of time, tetrahydrocannabinolic acid (THCA) will convert to cannabinolic acid (CBNA). CBN is then formed by decarboxylation of CBNA.
In contrast to THC, CBN has no double bond isomers nor stereoisomers. Both THC and CBN activate the CB1 and CB2 receptors.
CBN is not scheduled by the United Nations' Convention on Psychotropic Substances.
...
Wikipedia
