Cantharidin
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
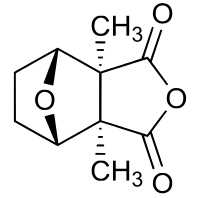
2,6-Dimethyl-4,10-dioxatricyclo-[5.2.1.02,6]decane-3,5-dione
|
|
| Other names
Cantharidin, Spanish Fly
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.240 |
| KEGG | |
| UNII | |
|
|
|
|
| Properties | |
| C10H12O4 | |
| Molar mass | 196.20 g·mol−1 |
| Density | 1.41 g/cm3 |
| Melting point | 212 °C (414 °F; 485 K) |
| Hazards | |
| Main hazards | Highly toxic |
| GHS pictograms |  |
|
EU classification (DSD)
|
|
| R-phrases | R28-R36/37/38 |
| S-phrases | S53-S45 |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
0.03–0.5 mg/kg (human) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Cantharidin is an odorless, colorless terpenoid secreted by many species of blister beetles, including broadly in genus Epicauta, and in species Lytta vesicatoria (Spanish fly). False blister beetles, cardinal beetles, and soldier beetles also produce cantharidin. Poisoning from the substance is a significant veterinary concern, especially in horses from the Epicauta species, but it can also be poisonous to humans if taken internally (where the origin is most often experimental self-exposure). Externally, cantharidin is a potent vesicant (blistering agent), exposure to which can cause severe chemical burns. Properly dosed and applied, the same properties have been used for effective topical medications for some conditions, such as treating patients with Molluscum contagiosum infection of the skin.
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
Cantharidin, from the Greek kantharis, for beetle, is an odorless, colorless natural product with solubility in various organic solvents, but only slightly solubility in water. It is a monoterpene, and so contains in its framework two isoprene units derived by biosynthesis from two equivalents of isopentenyl pyrophosphate. The complete mechanism of the biosynthesis of cantharidin is unknown. Its skeleton is tricyclic, formally, a tricyclo-[5.2.1.02,6]decane skeleton. Its functionalities include an carboxylic acid anhydride (-CO-O-CO-) substructure in one of its rings, as well as a cyclic ether in its bicyclic ring system.
...
Wikipedia

