Carbon tetraiodide
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Tetraiodomethane
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| 1733108 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.335 | ||
| EC Number | 208-068-5 | ||
|
PubChem CID
|
|||
| RTECS number | FG4960000 | ||
|
|||
|
|||
| Properties | |||
| CI4 | |||
| Molar mass | 519.63 g·mol−1 | ||
| Appearance | Dark violet crystals | ||
| Density | 4.32 g mL−1 | ||
| -136·10−6 cm3/mol | |||
| Structure | |||
| Tetragonal | |||
| Tetrahedral | |||
| 0 D | |||
| Thermochemistry | |||
| 0.500 J K−1 g−1 | |||
|
Std enthalpy of
formation (ΔfH |
384.0–400.4 kJ mol−1 | ||
|
Std enthalpy of
combustion (ΔcH |
−794.4–−778.4 kJ mol−1 | ||
| Hazards | |||
| GHS pictograms |  |
||
| GHS signal word | WARNING | ||
| H315, H319, H335 | |||
| P261, P305+351+338 | |||
|
EU classification (DSD)
|
|||
| R-phrases | R36/37/38 | ||
| S-phrases | S26, S36 | ||
| Related compounds | |||
|
Related alkanes
|
|||
|
Related compounds
|
|||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
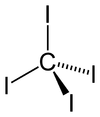
Carbon tetraiodide is a tetrahalomethane with the molecular formula CI4. Being bright red, it is a relatively rare example of a highly colored methane derivative. It is only 2% by weight carbon, although other methane derivatives are known with still less carbon.
The tetrahedral molecule features C-I distances of 2.12 ± 0.02 Å. The molecule is slightly crowded with short contacts between iodine atoms of 3.459 ± 0.03 Å, and possibly for this reason, it is thermally and photochemically unstable. Hexaiodoethane is unknown, probably for the same reason.
Carbon tetraiodide crystallizes in tetragonal crystal structure (a 6.409, c 9.558 (.10−1 nm)).
It has zero dipole moment due to its symmetrically substituted tetrahedral molecule.
Carbon tetraiodide is slightly reactive towards water, giving iodoform and I2>. Otherwise it is soluble in nonpolar organic solvents. It decomposes thermally and photochemically to tetraiodoethylene, C2I4. Its synthesis entails AlCl3-catalyzed halide exchange, which is conducted at room temperature:
The product crystallizes from the reaction solution.
Carbon tetraiodide is used as an iodination reagent, often upon reaction with bases. Ketones are converted to 1,1-diiodoalkenes upon treatment with triphenylphosphine (PPh3) and carbon tetraiodide. Alcohols are converted in and to iodide, by a mechanism similar to the Appel reaction. In an Appel reaction, carbon tetrachloride is used to generate alkyl chlorides from alcohols.
Manufacturers recommend that carbon tetraiodide be stored near 0 °C (32 °F). As a ready source of iodine, it is an irritant. Its LD50 is 178 mg.kg−1. In general, perhalogenated organic compounds should be considered toxic, with the narrow exception of small perfluoroalkanes (essentially inert due to the strength of the C-F bond).
...
Wikipedia


