Constella
|
|
| Clinical data | |
|---|---|
| Trade names | Linzess |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
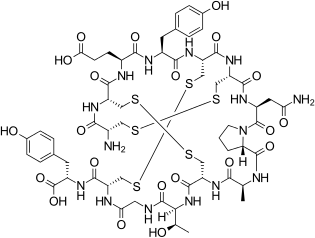
| Formula | C59H79N15O21S6 |
| Molar mass | 1526.74 g/mol |
| 3D model (JSmol) | |
|
|
|
|
|
|
|
Linaclotide (marketed under the trade name Linzess in the US and Mexico, and as Constella in Canada and many other countries) is an oligo-peptide agonist of guanylate cyclase 2C. It was approved by the FDA in August 2012 and by the European Medicines Agency for the treatment in adults of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation.
The National Institutes of Health (NIH) estimate that as many as 20% of Americans may experience irritable bowel syndrome (IBS); approximately one-third of those, as many as 10 million Americans, experience constipation-predominant IBS (IBS-C), that is, IBS with constipation often accompanied by abdominal pain.Laxatives can assist with constipation but do not treat pain, while opiates that are often prescribed to treat pain can aggravate constipation. While low-cost laxatives and pain killers would likely be tried first, linaclotide targets both associated conditions in a once-daily pill.
Linaclotide is a peptide mimic of endogenous guanylin and uroguanylin. It is a synthetic tetradecapeptide (14 amino acid peptide) with the sequence CCEYCCNPACTGCY by one-letter abbreviation, or by three-letter abbreviation:
H–Cys1–Cys2–Glu3–Tyr4–Cys5–Cys6–Asn7–Pro8–Ala9–Cys10–Thr11–Gly12–Cys13–Tyr14–OH
...
Wikipedia
