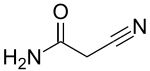
Cyanoacetamide
 |
|
| Names | |
|---|---|
|
IUPAC name
2-Cyanoacetamide
|
|
| Other names
Malonamide nitrile
3-Nitrilopropionamide |
|
| Identifiers | |
|
107-91-5 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider | 7610 |
| EC Number | 203-531-8 |
| UNII |
YBK38G2YXH |
|
|
|
|
| Properties | |
| C3H4N2O | |
| Molar mass | 84.08 g·mol−1 |
| Density | 1.163 g/cm3 |
| Melting point | 119 to 121 °C (246 to 250 °F; 392 to 394 K) |
| Boiling point | 351.2 °C (664.2 °F; 624.3 K) |
| Hazards | |
| R-phrases | R8 R23/24/25 R36/38 R45 |
| S-phrases | S17 S45 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
2-Cyanoacetamide is a organic compound. It is an acetic amide with a nitrile functional group.
Cyanoacetamide is used in spectrofluorimetric methods to determine the activity of antihistamine H1 receptor antagonistic drugs such as ebastine, cetirizine dihydrochloride and fexofenadine hydrochloride.
2-Cyanoacetamide is prepared from chloroacetic acid via Kolbe nitrile synthesis followed by Fischer esterification and ester aminolysis.
...
Wikipedia
