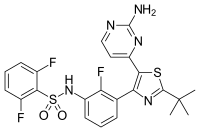
Dabrafenib
 |
|
| Clinical data | |
|---|---|
| Trade names | Tafinlar |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.215.965 |
| Chemical and physical data | |
| Formula | C23H20F3N5O2S2 |
| Molar mass | 519.56 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Dabrafenib (trade name Tafinlar, GSK2118436) is a drug for the treatment of cancers associated with a mutated version of the gene BRAF. Dabrafenib acts as an inhibitor of the associated enzyme B-Raf, which plays a role in the regulation of cell growth. Dabrafenib has clinical activity with a manageable safety profile in clinical trials of phase 1 and 2 in patients with BRAF(V600)-mutated metastatic melanoma.
The Food and Drug Administration initially approved dabrafenib as a single agent treatment for patients with BRAF V600E mutation-positive advanced melanoma on May 30, 2013. Clinical trial data demonstrated that resistance to dabrafinib and other BRAF inhibitors occurs within 6 to 7 months. To overcome this resistance, the BRAF inhibitor dabrafenib was combined with the MEK inhibitor trametinib. On January 8, 2014, the FDA approved this combination of dabrafenib and trametinib for BRAF V600E/K-mutant metastatic melanoma.
...
Wikipedia
