Dimethylmagnesium
 |
|
| Names | |
|---|---|
|
IUPAC name
Dimethylmagnesium
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C2H6Mg | |
| Molar mass | 54.38 g·mol−1 |
| Density | 0.96 g/cm3 |
| Reacts | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Dimethylmagnesium is an organomagnesium compound. Like other dialkylmagnesium compounds, it is prepared by adding at least one equivalent of dioxane to a solution of methylmagnesium halide:
The complex of magnesium dihalide and dioxane precipitates as a solid, driving the Schlenk equilibrium to give the dialkylmagnesium compound, which remains in solution.
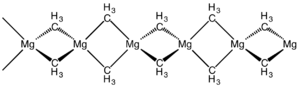
The structure of this compound has been determined by X-ray crystallography. The material is a polymer with tetrahedral magnesium centres, each surrounded by bridging methyl groups. The Mg-C distances are 224 pm.
...
Wikipedia
