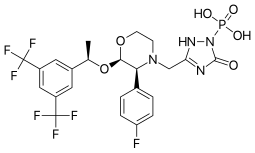
Fosaprepitant
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Multum Consumer Information |
| MedlinePlus | a604003 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | >95% (aprepitant) |
| Metabolism | To aprepitant |
| Biological half-life | 9 to 13 hours (aprepitant) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H22F7N4O6P |
| Molar mass | 614.406 g/mol |
| 3D model (JSmol) | |
|
|
|
|
|
|
|
Fosaprepitant (Emend for Injection (US), Ivemend (EU)) is an antiemetic drug, administered intravenously. It is a prodrug of aprepitant.
Fosaprepitant was developed by Merck & Co. and was approved by the United States Food and Drug Administration (FDA) on January 25, 2008 and by the European Medicines Agency (EMA) on January 11 of the same year.
...
Wikipedia
