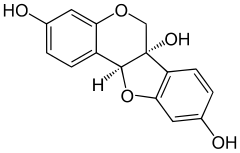
Glycinol (pterocarpan)
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
6H-[1]benzofuro[3,2-c]chromene-3,6a(11aH),9-triol
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C15H12O5 | |
| Molar mass | 272.25 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Glycinol is a pterocarpan, a type of natural phenol. It is a phytoalexin found in the soybean (Glycine max). It is formed by the cyclisation of daidzein. It has antiestrogenic activities.
It can be synthethised chemically and possesses two chiral centers.
Glycinol is the direct precursor of glyceollins through the action of a prenyltransferase.
Experiments show that the 6a oxygen of glycinol is derived from molecular oxygen.
...
Wikipedia
