Guanine
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
2-amino-9H-purin-6(1H)-one
|
|||
| Other names
1,9-dihydro-6H-purin-6-one,
2-amino-6-hydroxypurine, 2-aminohypoxanthine, Guanine |
|||
| Identifiers | |||
|
73-40-5 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:16235 |
||
| ChEMBL |
ChEMBL219568 |
||
| ChemSpider |
744 |
||
| DrugBank |
DB02377 |
||
| ECHA InfoCard | 100.000.727 | ||
| 4556 | |||
| KEGG |
C00242 |
||
| RTECS number | MF8260000 | ||
| UNII |
5Z93L87A1R |
||
|
|||
|
|||
| Properties | |||
| C5H5N5O | |||
| Molar mass | 151.13 g/mol | ||
| Appearance | White amorphous solid. | ||
| Density | 2.200 g/cm3 (calculated) | ||
| Melting point | 360 °C (680 °F; 633 K) decomposes | ||
| Boiling point | Sublimes | ||
| Insoluble. | |||
| Acidity (pKa) | 3.3 (amide), 9.2 (secondary), 12.3 (primary) | ||
| Hazards | |||
| Main hazards | Irritant | ||
| NFPA 704 | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
|
Related compounds
|
Cytosine; Adenine; Thymine; Uracil | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Guanine (/ˈɡwɑːnᵻn/; or G, Gua) is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine (uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside is called guanosine.
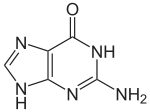
With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with conjugated double bonds. Being unsaturated, the bicyclic molecule is planar.
Guanine, along with adenine and cytosine, is present in both DNA and RNA, whereas thymine is usually seen only in DNA, and uracil only in RNA. Guanine has two tautomeric forms, the major keto form (see figures) and rare enol form. It binds to cytosine through three hydrogen bonds. In cytosine, the amino group acts as the hydrogen bond donor and the C-2 carbonyl and the N-3 amine as the hydrogen-bond acceptors. Guanine has the C-6 carbonyl group that acts as the hydrogen bond acceptor, while a group at N-1 and the amino group at C-2 act as the hydrogen bond donors.
Guanine can be hydrolyzed with strong acid to glycine, ammonia, carbon dioxide, and carbon monoxide. First, guanine gets deaminated to become xanthine. Guanine oxidizes more readily than adenine, the other purine-derivative base in DNA. Its high melting point of 350 °C reflects the intermolecular hydrogen bonding between the oxo and amino groups in the molecules in the crystal. Because of this intermolecular bonding, guanine is relatively insoluble in water, but it is soluble in dilute acids and bases.
...
Wikipedia



