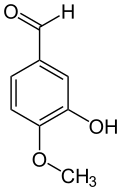
Isovanillin
 |
|
| Names | |
|---|---|
|
Systematic IUPAC name
3-Hydroxy-4-methoxybenzaldehyde
|
|
| Other names
5-Formylguaiacol
3-Hydroxy-p-anisaldehyde |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| 1073021 | |
| ChemSpider | |
| ECHA InfoCard | 100.009.724 |
| EC Number | 210-694-9 |
| MeSH | Isovanillin |
|
PubChem CID
|
|
| RTECS number | CU6540000 |
|
|
|
|
| Properties | |
| C8H8O3 | |
| Molar mass | 152.15 g·mol−1 |
| Appearance | Translucent crystals |
| Melting point | 113 to 116 °C (235 to 241 °F; 386 to 389 K) |
| Boiling point | 179 °C (354 °F; 452 K) at 15 mmHg |
| log P | 1.25 |
| Acidity (pKa) | 9.248 |
| Hazards | |
|
EU classification (DSD)
|
|
| R-phrases | R36/37/38 |
| S-phrases | S26, S36/37 |
| Related compounds | |
|
Related compounds
|
Anisaldehyde |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
3-Hydroxy-p-anisaldehyde
3-Hydroxy-4-methoxybenzaldehyde
Isovanillin is a phenolic aldehyde, an organic compound and isomer of vanillin. It is a selective inhibitor of aldehyde oxidase. It is not a substrate of that enzyme, and is metabolized by aldehyde dehydrogenase into isovanillic acid. Isovanillin can be used as a precursor in the total synthesis of morphine.
...
Wikipedia
