L-type lectin domain
| Lectin_leg-like | |||||||||
|---|---|---|---|---|---|---|---|---|---|
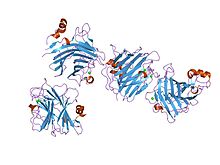
the crystal structure of the carbohydrate recognition domain of the glycoprotein sorting receptor p58/ergic-53 reveals a novel metal binding site and conformational changes associated with calcium ion binding
|
|||||||||
| Identifiers | |||||||||
| Symbol | Lectin_leg-like | ||||||||
| Pfam | PF03388 | ||||||||
| Pfam clan | CL0004 | ||||||||
| InterPro | IPR005052 | ||||||||
| SCOP | 1gv9 | ||||||||
| SUPERFAMILY | 1gv9 | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
In molecular biology the L-like lectin domain is a protein domain found in lectins which are similar to the leguminous plant lectins.
Lectins are structurally diverse proteins that bind to specific carbohydrates. This family includes the VIP36 and ERGIC-53 lectins. Although proteins containging this domain were originally identified as a family of animal lectins, there are also yeast representatives.
ERGIC-53 is a 53kDa protein, localised to the intermediate region between the endoplasmic reticulum and the Golgi apparatus (ER-Golgi-Intermediate Compartment, ERGIC). It was identified as a calcium-dependent, mannose-specific lectin. Its has been associated with combined factors V and VIII deficiency, suggesting an important and substrate-specific role for ERGIC-53 in the glycoprotein-secreting pathway.
The L-like lectin domain has an overall globular shape composed of a beta-sandwich of two major twisted antiparallel beta-sheets. The beta-sandwich comprises a major beta-sheet and a minor beta-sheet, in a variation of the jelly roll fold.
This article incorporates text from the public domain Pfam and InterPro IPR005052
...
Wikipedia
