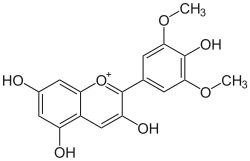
Malvidin
 |
|
| Names | |
|---|---|
|
IUPAC name
3,5,7-trihydroxy-2-(4-hydroxy- 3,5-dimethoxyphenyl)chromenium
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.368 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C17H15O7+ | |
| Molar mass | 331.2968 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Malvidin (Mv) is an O-methylated anthocyanidin. As a primary plant pigment, its glycosides are highly abundant in nature.
Malvidin is responsible for the blue color found in petals of the Primula plants of the polyanthus group. Blue flowers of the blue pimpernel (Anagallis monelli) have also a higher concentration of malvidin.
It is responsible primarily for the color of red wine, Vitis vinifera being one of its sources. It is also present in other berries, such as the chokeberries (Aronia sp.) or the saskatoon berries (Amelanchier alnifolia).
Slightly acidic and neutral solutions of malvidin are characteristically of a red color, while basic solutions of malvidin yield a blue color.
The breakdown of malvidin releases syringic acid.
The breakdown of malvidin releases syringic acid as revealed in the examination of jars containing shedeh, a drink of Ancient Egypt. Malvidin is also present in the site of the Areni-1 winery, a 6,100-year-old winery discovered in 2007 in the Areni-1 cave complex in the village of Areni in the Vayots Dzor province of the Republic of Armenia.
...
Wikipedia
