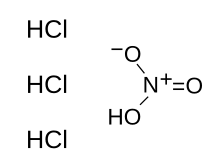
Nitromuriatic acid
 |
|
| Names | |
|---|---|
|
IUPAC name
nitric acid hydrochloride
|
|
| Other names
aqua regis, nitrohydrochloric acid
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
|
PubChem CID
|
|
|
|
| Properties | |
| HNO3+3 HCl | |
| Appearance | red, yellow or gold fuming liquid |
| Density | 1.01–1.21 g/cm3 |
| Melting point | −42 °C (−44 °F; 231 K) |
| Boiling point | 108 °C (226 °F; 381 K) |
| miscible in water | |
| Vapor pressure | 21 mbar |
| Hazards | |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Aqua regia /ˈa.kʷə ˈɹeɪ.gi.ə/ (from Latin, lit. "royal water" or "king's water") is a mixture of nitric acid and hydrochloric acid, optimally in a molar ratio of 1:3. Aqua regia is a yellow-orange fuming liquid, so named by alchemists because it can dissolve the noble metals gold and platinum, though not all metals.
Aqua regia is primarily used to produce chloroauric acid, the electrolyte in the Wohlwill process for refining the highest quality (99.999%) gold.
Aqua regia is also used in etching and in specific analytic procedures. It is also used in some laboratories to clean glassware of organic compounds and metal particles. This method is preferred over the more traditional chromic acid bath for cleaning NMR tubes, because no traces of paramagnetic chromium can remain to spoil spectra. While chromic acid baths are discouraged because of the high toxicity of chromium and the potential for explosions, aqua regia is itself very corrosive and has been implicated in several explosions due to mishandling.
Due to the reaction between its components resulting in its decomposition, aqua regia quickly loses its effectiveness (yet remains a strong acid), so its components are usually only mixed immediately before use.
...
Wikipedia

