Pivagabine
 |
|
| Names | |
|---|---|
|
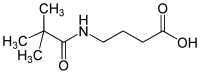
IUPAC name
4-(2,2-Dimethylpropanoylamino)butanoic acid
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.067.287 |
| EC Number | 274-038-3 |
| KEGG | |
| MeSH | N-trimethylacetyl-4-aminobutyric+acid |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C9H17NO3 | |
| Molar mass | 187.24 g·mol−1 |
| Pharmacology | |
| N06AX15 (WHO) | |
| Oral | |
| Pharmacokinetics: | |
| 6.4 hours | |
| Legal status |
|
| Related compounds | |
|
Related alkanoic acids
|
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Pivagabine (INN; brand name Tonerg), also known as N-pivaloyl-γ-aminobutyric acid or N-pivaloyl-GABA, is an antidepressant and anxiolytic drug which was introduced in Italy in 1997 for the treatment of depressive and maladaptive syndromes. But it was discontinued in Italy (according to Martindale). Originally believed to function as a prodrug to GABA, pivagabine is now believed to act somehow via modulation of corticotropin-releasing factor (CRF).
...
Wikipedia
