Pyrocitric acid
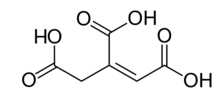
cis-aconitic acid
|
|

trans-aconitic acid
|
|
| Names | |
|---|---|
|
IUPAC name
Prop-1-ene-1,2,3-tricarboxylic acid
|
|
| Other names
achilleic acid; equisetic acid; citridinic acid; pyrocitric acid, achilleaic acid
|
|
| Identifiers | |
| ChemSpider |
|
| ECHA InfoCard | 100.007.162 |
|
PubChem CID
|
|
|
|
| Properties | |
| C6H6O6 | |
| Molar mass | 174.11 g·mol−1 |
| Appearance | Colorless crystals |
| Melting point | 190 °C (374 °F; 463 K) (decomposes) (mixed isomers), 173 °C (cis and trans isomers) |
| Acidity (pKa) | 2.80, 4.46 (trans isomer) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Aconitic acid is an organic acid. The two isomers are cis-aconitic acid and trans-aconitic acid. The conjugate base of cis-aconitic acid, cis-aconitate is an intermediate in the isomerization of citrate to isocitrate in the citric acid cycle. It is acted upon by the enzyme aconitase.
Aconitic acid can be synthesized by dehydration of citric acid using sulfuric acid:
A mixture of isomers are generated in this way.
It was first prepared by thermal dehydration.
...
Wikipedia
