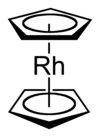
Rhodocene
 |
|
| Names | |
|---|---|
|
IUPAC name
bis(η5-cyclopentadienyl)rhodium(II)
|
|
| Other names
rhodocene
dicyclopentadienylrhodium |
|
| Identifiers | |
|
12318-21-7 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
2339512 |
| PubChem | 3082022 |
|
|
|
|
| Properties | |
| C10H10Rh | |
| Molar mass | 233.10 g·mol−1 |
| Appearance | yellow solid (dimer) |
| Melting point | 174 °C (345 °F; 447 K) with decomposition (dimer) |
| somewhat soluble in dichloromethane (dimer) soluble in acetonitrile |
|
| Related compounds | |
|
Related compounds
|
ferrocene, , iridocene, bis(benzene)chromium |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Rhodocene, formally known as bis(η5-cyclopentadienyl)rhodium(II), is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C or when trapped by cooling to liquid nitrogen temperatures (−196 °C). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
The history of organometallic chemistry includes the 19th-century discoveries of Zeise's salt and nickel tetracarbonyl. These compounds posed a challenge to chemists as the compounds did not fit with existing chemical bonding models. A further challenge arose with the discovery of ferrocene, the iron analogue of rhodocene and the first of the class of compounds now known as metallocenes. Ferrocene was found to be unusually chemically stable, as were analogous chemical structures including rhodocenium, the unipositive cation of rhodocene and its cobalt and iridium counterparts. The study of organometallic species including these ultimately led to the development of new bonding models that explained their formation and stability. Work on sandwich compounds, including the rhodocenium-rhodocene system, earned Geoffrey Wilkinson and Ernst Otto Fischer the 1973 Nobel Prize for Chemistry.
...
Wikipedia
