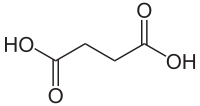
Succinate
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Butanedioic acid
|
|
| Other names
Succinic acid
Ethane-1,2-dicarboxylic acid |
|
| Identifiers | |
|
110-15-6 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:15741 |
| ChEMBL |
ChEMBL576 |
| ChemSpider |
1078 |
| DrugBank |
DB00139 |
| ECHA InfoCard | 100.003.402 |
| E number | E363 (antioxidants, ...) |
| 3637 | |
| PubChem | 1110 |
| UNII |
AB6MNQ6J6L |
|
|
|
|
| Properties | |
| C4H6O4 | |
| Molar mass | 118.09 g·mol−1 |
| Density | 1.56 g/cm3 |
| Melting point | 184 °C (363 °F; 457 K) |
| Boiling point | 235 °C (455 °F; 508 K) |
| 58 g/L (20 °C) or 100 mg/mL | |
| Solubility in Methanol | 158 mg/mL |
| Solubility in Ethanol | 54 mg/mL |
| Solubility in Acetone | 27 mg/mL |
| Solubility in Glycerol | 50 mg/mL |
| Solubility in Ether | 8.8 mg/mL |
| Acidity (pKa) | pKa1 = 4.2 pKa2 = 5.6 |
| -57.9·10−6 cm3/mol | |
| Hazards | |
| Flash point | 206 °C (403 °F; 479 K) |
| Related compounds | |
|
Other anions
|
sodium succinate |
|
Related carboxylic acids
|
propionic acid malonic acid butyric acid malic acid tartaric acid fumaric acid valeric acid glutaric acid |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Succinic acid (/səkˈsɪnᵻk/) is a dicarboxylic acid with chemical formula (CH2)2(CO2H)2. It is a white, odorless solid. In an aqueous solution, it ionizes to anions (that is, conjugates to a conjugate base) called succinate (/ˈsʌksᵻneɪt/), which plays a role in the citric acid cycle, an energy-yielding process in all living organisms. As a radical group it is called a succinyl (/ˈsʌksᵻnəl/) group. The name derives from Latin succinum, meaning amber, from which the acid may be obtained.
Common industrial routes include partial hydrogenation of maleic acid, oxidation of 1,4-butanediol, and carbonylation of ethylene glycol.
...
Wikipedia
