Sulfide ore
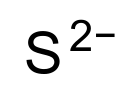 |
|
| Names | |
|---|---|
|
Systematic IUPAC name
Sulfanediide (substitutive)
Sulfide(2−) (additive) |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| S2− |
|
| Molar mass | 32.06 g·mol−1 |
| Related compounds | |
|
Other anions
|
Telluride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Sulfide (systematically named sulfanediide, and sulfide(2−)) (British English sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. It contributes no color to sulfide salts. As it is classified as a strong base, even dilute solutions of salts such as sodium sulfide (Na2S) are corrosive and can attack the skin. Sulfide is the simplest sulfur anion.
The systematic names sulfanediide and sulfide(2−), valid IUPAC names, are determined according to the substitutive and additive nomenclatures, respectively. However, the name sulfide is also used in compositional IUPAC nomenclature which does not take the nature of bonding involved. Examples of such naming are selenium disulfide and titanium sulfide, which contains no sulfide ions whatsoever.
Sulfide is also used non-systematically, to describe compounds which release hydrogen sulfide upon acidification, or a compound that otherwise incorporates sulfur in some form, such as dimethyl sulfide. "Hydrogen sulfide" is itself an example of a non-systematic name of this nature. However, it is also a trivial name, and the preferred IUPAC name for sulfane.
It has been confirmed that the sulfide ion, S2-, does not exist even in hyper-concentrated aqueous alkaline solutions of Na2S. Thus, the dissociation reaction
does not occur in aqueous solution at any concentration of sulphide. The sulphide ion, S2−, was previously reported to be undetectable at concentrations up to 5M NaOH. However, the sulfide ion may be produced when a solid is formed. For example cadmium sulphide precipitates in group 2 of qualitative analysis.
...
Wikipedia
