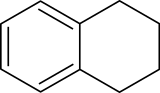
Tetralin
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
1,2,3,4-tetrahydronaphthalene
|
|
| Other names
naphthalene 1,2,3,4-tetrahydride
Bacticin benzocyclohexane THN |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.946 |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C10H12 | |
| Molar mass | 132.21 g·mol−1 |
| Appearance | Clear, colorless liquid with an odor similar to naphthalene |
| Density | 0.970 g/cm3 |
| Melting point | −35.8 °C (−32.4 °F; 237.3 K) |
| Boiling point | 206 to 208 °C (403 to 406 °F; 479 to 481 K) |
| Insoluble | |
| Viscosity | 2.02 cP at 25 °C |
| Hazards | |
| Safety data sheet | JT Baker MSDS |
| Flash point | 77 °C (171 °F; 350 K) |
| 385 °C (725 °F; 658 K) | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Tetralin (1,2,3,4-tetrahydronaphthalene) is a hydrocarbon having the chemical formula C10H12. This molecule is similar to the naphthalene chemical structure except that one ring is saturated.
The compound can be synthesized in a Bergman cyclization whereby cyclodeca-3-ene-1,5-diyne reacts with 1,3-cyclohexadiene to produce benzene and tetralin. In a classic named reaction called the Darzens tetralin synthesis, named for Auguste Georges Darzens (1926), derivatives can be prepared by intramolecular electrophilic aromatic substitution reaction of an 1-aryl-4-pentene using concentrated sulfuric acid,
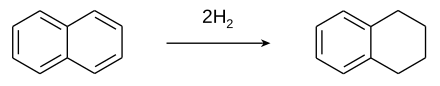
It can also be prepared by partial hydrogenation of naphthalene in the presence of a platinum catalyst.
Tetralin is produced by the catalytic hydrogenation of naphthalene.
...
Wikipedia