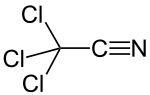
Trichloroacetonitrile
 |
|
| Names | |
|---|---|
|
IUPAC name
Trichloroacetonitrile
|
|
| Identifiers | |
| 545-06-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 13861934 |
| ECHA InfoCard | 100.008.078 |
| PubChem | 24900271 |
|
|
|
|
| Properties | |
| C2Cl3N | |
| Molar mass | 144.38 g·mol−1 |
| Appearance | colourless liquid |
| Density | 1.44 g/mL |
| Melting point | -42 |
| Boiling point | 83 to 84 °C (181 to 183 °F; 356 to 357 K) |
| insoluble | |
| Hazards | |
| Main hazards | GHS06, GHS09 |
| Safety data sheet | MSDS |
| NFPA 704 | |
| Flash point | 195 °C (383 °F; 468 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Trichloroacetonitrile is an organic compound with the formula CCl3CN. It is a colourless liquid, although commercial samples often are brownish. It is used commercially as a precursor to the fungicide etridiazole. It is prepared by dehydration of trichloroacetamide.
In academic research, trichloroacetonitrile is used as a reagent in the Overman rearrangement, converting allylic alcohols into allylic amines.
...
Wikipedia

