Wagner-Meerwein rearrangement
A Wagner–Meerwein rearrangement is a class of carbocation 1,2-rearrangement reactions in which a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon.
Several reviews have been published.
The rearrangement was first discovered in bicyclic terpenes for example the conversion of isoborneol to camphene:
The story of the rearrangement reveals that many scientists were puzzled with this and related reactions and its close relationship to the discovery of carbocations as intermediates.
In a simple demonstration reaction of 1,4-dimethoxybenzene with either 2-methyl-2-butanol or 3-methyl-2-butanol in sulfuric acid and acetic acid yields the same disubstituted product, the latter via a hydride shift of the cationic intermediate:
Currently, there are works relating to the use of skeletal rearrangement in the synthesis of bridged azaheterocycles. These data are summarized in
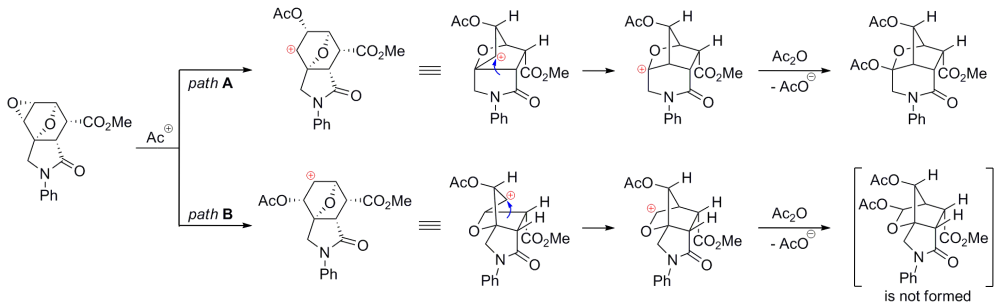
Plausible mechanisms of the Wagner–Meerwein rearrangement of diepoxyisoindoles:
...
Wikipedia