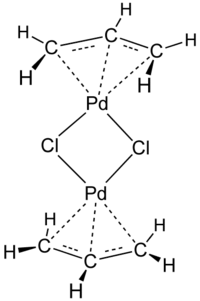
Allyl palladium chloride
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Allylpalladium(II) chloride dimer
|
|
| Other names
Allylpalladium chloride dimer
bis(allyl)di-μ-chloro-dipalladium(II) APC |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.423 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C6H10Cl2Pd2 | |
| Molar mass | 365.85 g/mol |
| Appearance | Pale yellow, crystalline solid |
| Density | Solid |
| Melting point | decomp at 155-156 °C |
| Insoluble | |
| Solubility in other solvents |
Chloroform benzene acetone methanol |
| Hazards | |
| Safety data sheet | http://www.colonialmetals.com/pdf/5048.pdf |
| R-phrases (outdated) | 36/37/38 |
| S-phrases (outdated) | 26-36 |
| Related compounds | |
|
Related compounds
|
(η3-allyl)(η5 – cyclopentadienyl)palladium(II) di-μ-chlorobis(crotyl)dipalladium |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Allylpalladium(II) chloride dimer (APC) is a chemical compound with the formula [(η3-C3H5)PdCl]2. This yellow air-stable compound is an important catalyst used in organic synthesis. It is one of the most widely used transition metal allyl complexes.
The compound is prepared by purging carbon monoxide through a methanolic aqueous solution of sodium tetrachloropalladate (prepared from palladium(II) chloride and sodium chloride), and allyl chloride.
APC reacts with sources of cyclopentadienyl anion to give the corresponding 18e complex cyclopentadienyl allyl palladium:
...
Wikipedia
