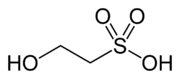
Isethionic acid
 |
|
 Isethionic acid
|
|
| Names | |
|---|---|
|
IUPAC name
2-hydroxyethanesulfonic acid
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.169 |
| KEGG | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C2H6O4S | |
| Molar mass | 126.13 g/mol |
| Density | 1.63 g/cm3 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Isethionic acid is an organosulfur compound containing a alkylsulfonic acid located beta to a hydroxy group. Its discovery is generally attributed to Heinrich Gustav Magnus, who prepared it by the action of solid sulphur trioxide on ethanol in 1833. It is a white water-soluble solid used in the manufacture of certain surfactants and in the industrial production of taurine. It is most commonly available in the form of its sodium salt (sodium isethionate).
Its original synthesis, by the reaction of sulfur trioxide on ethanol, has largely been surpassed. It may be produced by the hydrolysis of carbyl sulfate, which is obtained by the sulfonation of ethylene.
However the most common route is the reaction of ethylene oxide with aqueous sodium bisulfite, which produces the sodium salt (sodium isethionate):
Isethionic acid is used as a starting material in the industrial production of taurine.
Dehydration of isethionic acid gives vinylsulfonic acid.
Fatty acid esters of isethionic acid (such as sodium lauroyl isethionate and sodium cocoyl isethionate) are used as biodegradable anionic surfactants. These materials are much milder to skin that other sulphate based surfactants (i.e. sodium lauryl sulfate) making them popular for use in make-up, shampoos and ‘Dove type’ soap bars.
...
Wikipedia
