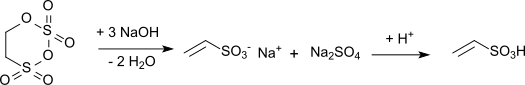Vinylsulfonic acid
 |
|
| Names | |
|---|---|
|
IUPAC name
ethenesulfonic acid
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.342 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C2H4O3S | |
| Molar mass | 108.11 g·mol−1 |
| Appearance | colouless liquid |
| Boiling point | 128 °C (1,29 hPa)* 95 °C |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Vinylsulfonic acid is the organosulfur compound with the formula CH2=CHSO3H. It is simplest unsaturated sulfonic acid. The C=C double bond is a site of high reactivity. polymerize gives polyvinylsulfonic acid, especially when used as a comonomer with functionalized vinyl and (meth)acrylic acid compounds. It is a colorless, water-soluble liquid, although commercial samples can appear yellow or even red.
Vinylsulfonic acid is produced industrially by the alkaline hydrolysis of carbyl sulfate with subsequent acidification of the resulting vinyl sulfonate salt:
The reaction is highly exothermic (reaction enthalpy: 1,675 kJ/kg) and requires exact maintenance of temperature and pH during the hydrolysis. When calcium hydroxide is used as the hydrolysis medium, a solution of calcium vinyl sulfonate is obtained. Acidification of this hydrolysis mixture with sulfuric acid gives vinylsulfonic acid, together with the poorly soluble calcium sulfate.
Vinylsulfonic acid also can be prepared by dehydration of isethionic acid with phosphorus pentoxide:
...
Wikipedia