L-leucine
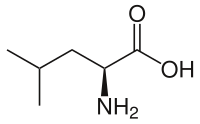
L-Leucine
|
|

L-Leucine at physiological pH
|
|
| Names | |
|---|---|
|
IUPAC name
Leucine
|
|
| Other names
2-Amino-4-methylpentanoic acid
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.475 |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C6H13NO2 | |
| Molar mass | 131.18 g·mol−1 |
| Acidity (pKa) | 2.36 (carboxyl), 9.60 (amino) |
| -84.9·10−6 cm3/mol | |
| Supplementary data page | |
|
Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Leucine (abbreviated as Leu or L; encoded by the six codons UUA, UUG, CUU, CUC, CUA, and CUG) is an α-amino acid used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and an isobutyl side chain, classifying it as a nonpolar (at physiological pH) amino acid. It is essential in humans—meaning the body cannot synthesize it and thus must obtain from the diet.
Leucine is a major component of the subunits in ferritin, astacin, and other "buffer" proteins.
Leucine is used in the liver, adipose tissue, and muscle tissue. Adipose and muscle tissue use leucine in the formation of sterols. Combined leucine use in these two tissues is seven times greater than in the liver.
Leucine is rapidly taken up into the brain where astrocytes convert it to alpha-ketoisocaproate via transamination of alpha-ketoglutarate to glutamate. [reference: Yudoff, Mark. (Feb 01 2017) "Interactions in the Metabolism of Glutamate and the Branched-Chain Amino Acids and ketoacids in the CNS" Neurochem Res. 2017 January; 42(1): 10-18. doi:10.1007/s11064-016-2057-z. ]
Leucine is an essential amino acid in the diet of animals because they lack the complete enzyme pathway to synthesize it de novo from potential precursor compounds. Consequently, they must ingest it, usually as a component of proteins. Plants and microorganisms synthesize leucine from pyruvic acid with a series of enzymes:
Synthesis of the small, hydrophobic amino acid valine also includes the initial part of this pathway.
...
Wikipedia
