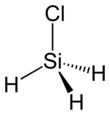
Silyl chloride
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Chlorosilane
|
|||
| Other names
Silyl chloride
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.033.354 | ||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| ClH3Si | |||
| Molar mass | 66.56 g·mol−1 | ||
| Solubility in ether and THF | Soluble | ||
| Hazards | |||
| Main hazards | flammable | ||
| Related compounds | |||
|
Related chlorosilanes
|
Dichlorosilane Trichlorosilane Silicon tetrachloride |
||
|
Related compounds
|
Chloromethane Iodosilane Methylsilane |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond.
They are prepared by the Müller-Rochow process, which involves treating silicon with hydrogen chloride at elevated temperatures in the presence of a copper catalyst. The idealized equation is
Trichlorosilane (HSiCl3) is the main product; dichlorosilane (H2SiCl2) and silicon tetrachloride (SiCl4) are obtained as byproducts. The process was independently discovered by Eugene G. Rochow and Richard Müller in 1940.
All chlorosilanes react with water to produce hydrogen chloride. The remaining hydroxyl group bonds to the silicon, initially forming a silanol group (analogous to alcohol). In general, this will eventually bond to a solid oxide surface or react with another chlorosilane or silol molecule. In the latter cases, the oxygen atom forms a link between two silicon atoms, analogous to the ether linkage in organic chemicals, and identical to the bonding in silicon dioxide.
Silicon tetrachloride (SiCl
4) and trichlorosilane (HSiCl
3) are intermediates in the production of ultrapure silicon in the semiconductor industry. Chlorosilanes obtained from crude silicon are purified by fractional distillation techniques and then reduced with hydrogen to give silicon of 99.999999999% purity.
...
Wikipedia